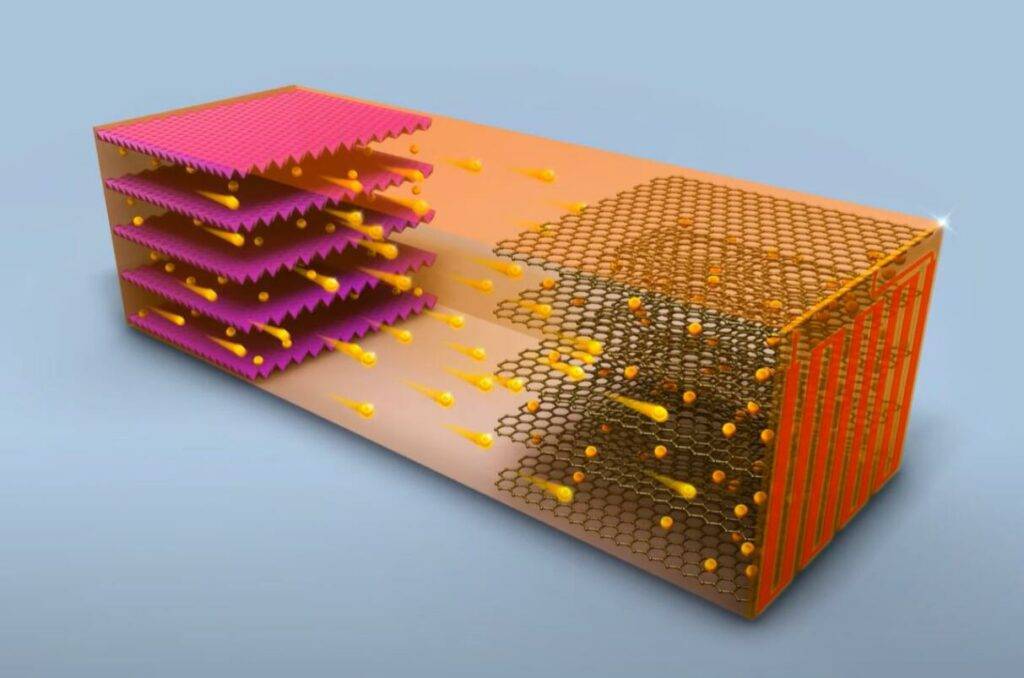
Substituting solid-state electrolytes is the next critical step towards advancing lithium-ion batteries with better thermal stability and chemical stability. Recently, researchers Michael J. Zdilla and Stephanie L. Wunder from Temple University, along with Arun Venkatnathan from the Indian Institute of Science Education and Research, reported a soft eutectic solid electrolyte for lithium-ion batteries.
#post_seo_title
Key points of the Research:
Synthesis and Characterization of (Adpn)2LiPF6 Electrolyte:
The authors synthesized and characterized a soft solid-state electrolyte, (Adpn)2LiPF6, where Adpn represents hexanenitrile.
Enhanced Ionic Conductivity and Stability:
This electrolyte exhibits high thermal and electrochemical stability and possesses good ionic conductivity, overcoming limitations of traditional organic and ceramic materials.
Novel Crystal Design for Improved Thermal Stability:
The electrolyte’s surface features a liquid-like nanolayer of Adpn that connects grains for ion conduction without requiring high pressure/temperature treatments. Furthermore, if the material fractures, it can rapidly self-heal and provide a liquid-like conduction pathway through grain boundaries.
Unique Ion Conduction Mechanism in Soft Solid-State Electrolytes:
This electrolyte demonstrates a relatively high ionic conductivity (~10^-4 S cm^-1) and lithium ion transference number (0.54). This is attributed to the weak interactions between the “hard” (charge-dense) Li+ ions and the “soft” (electron-polarizable) -C≡N groups of Adpn. Through molecular simulations, the authors discovered that Li+ ions migrate across the eutectic grain boundaries with lower activation energy (Ea) and within interstitial regions with higher Ea values between the eutectics. This work introduces a unique crystal design concept, enhancing the thermal stability of LiPF6 by isolating ions within an Adpn solvent matrix. It exhibits a distinctive ion conduction mechanism with low-resistance grain boundaries, in contrast to ceramic or gel electrolytes.